How is the clinical trial progressing?
2022-05-20
On May 31, 2022, Lipigon initiated the first clinical study of Lipisense®, with the first human ever receiving the drug candidate. Stefan Pierrou, the project management lead for drug development at Lipigon, provides insights into the significance of this event and what the future holds.

Lipigon's most advanced project, Lipisense®, aims to reduce levels of harmful blood fat triglycerides. In late spring, Lipisense® was administered to a human for the first time as part of the Phase I study of the drug candidate.
Stefan Pierrou, who leads this study, brings a wealth of experience in preclinical and clinical development, with 17 years of experience at AstraZeneca. Even though he has taken drug candidates into clinical phases many times before, he describes the feeling as remarkable.
"Projects of this magnitude require massive preparation, and many things can go wrong in the preclinical phase. However, after two years of dedicated work on Lipisense®, overcoming hurdles that, thankfully, were not insurmountable, we now have come out the other side. It feels incredibly exciting," says Stefan Pierrou.
Stefan Pierrou explains that working in a small company with few projects is different from large companies where 10-20 projects run in parallel.
"In a smaller company, you're deeply involved at every stage, resource optimization becomes paramount, and every contingency must be considered. This adds a great deal of excitement to the process," he further elaborates.
Designed Molecule with High Precision
Lipigon's drug candidate Lipisense® is an antisense oligonucleotide, or ASO. An ASO is a short DNA/RNA sequence that binds complementarily to a gene's mRNA, preventing cells from producing a protein. In this case, the target is the ANGPTL4 protein, which is precisely what researchers aim to inhibit. ANGPTL4 binds to lipoprotein lipase (LPL), a central enzyme responsible for breaking down fats in the blood. Inhibiting ANGPTL4 increases LPL activity, leading to greater fat breakdown.
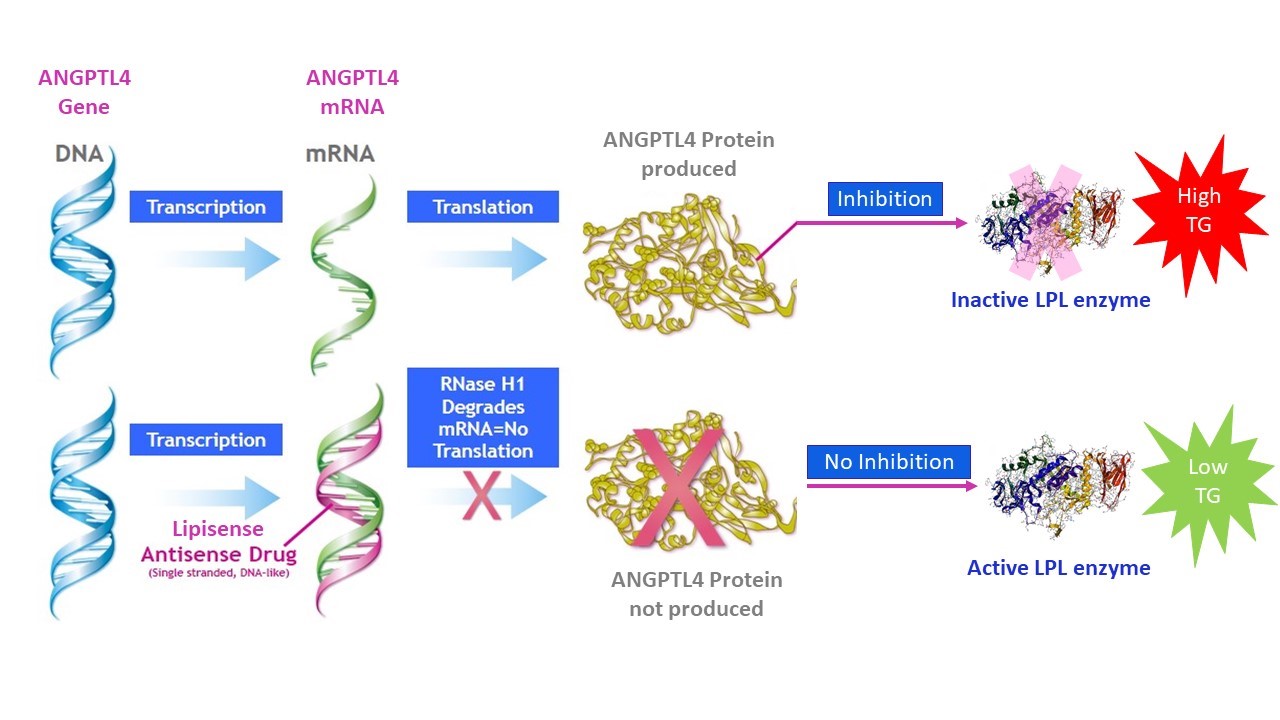
How Lipisense® operates:
Top Row: When a gene is transcribed, it generates a working copy of the DNA called mRNA. This mRNA acts as a template for protein synthesis, such as the ANGPTL4 protein. ANGPTL4 protein inhibits another protein, lipoprotein lipase (LPL), within the body. LPL plays a critical role in breaking down triglycerides in the bloodstream, and reduced LPL levels result in elevated blood lipid levels. Bottom Row: Conversely, when mRNA binds to Lipisense®, it prevents the production of the ANGPTL4 protein, leaving LPL unbound. As a result, LPL can continue circulating in the bloodstream, enhancing the breakdown of fats.
Antisense is a relatively new type of medication that, in certain cases, has advantages over drugs based on small molecules. The challenge is to develop molecules that do not activate the immune system in the wrong way while binding to mRNA correctly. In the case of Lipisense®, it involves 17 binding sites, known as base pairs, and researchers have tested over 60 different oligonucleotides in the preclinical phase to find the right one.
One of the key advantages of antisense drugs is their relatively low risk of side effects. They are more likely to affect solely the targeted gene, unlike small molecule drugs that can impact multiple processes in the body.
In Lipisense®'s case, researchers have also equipped the ASO with a specific group of three sugar molecules, making it liver-specific to avoid side effects that would occur if ANGPTL4 were inhibited throughout the entire body.
“It is only then that one truly grasps the long preclinical journey we've undertaken to prepare for this initial clinical study. Designing a molecule in this manner demands time and patience, so it's certainly remarkable that we've finally commenced human trials," Stefan Pierrou explains.
Safety and the Prospect of Effectiveness
The Phase I study is a randomized, double-blind, placebo-controlled study of single ascending doses (SAD) and multiple ascending doses (MAD).
This means the group receiving the drug is compared to a randomly selected control group. This is done to account for the placebo effect, where the mere belief in receiving an effective treatment leads to an effect. A double-blind study means neither the patient nor the hospital staff knows which treatment is given.
"Concretely, each test subject receives an injection just under the skin in the abdominal area, and then closely monitored for 32 hours, with frequent blood tests. Afterwards, there is a follow-up for 31 days. We examine how the drug is metabolized in the body and how blood levels change," explains Stefan Pierrou.
"The hypothesis is that if we inhibit ANGPTL4, LPL activity will increase, and blood triglyceride levels will decrease. If we can measure this in the SAD part of the study, it reduces a significant portion of the risk in the continued development program for Lipisense®, and we will know it works as intended."
Positive Data After Five Months
The SAD segment of the study initially encompassed 20 healthy participants divided into four groups, with the primary objective of evaluating the safety and tolerability of a Lipisense® injection or placebo. Initially, four different dose levels were assessed: 2, 6, 18, and 36 mg.

Extensive Preparations
Lipigon was founded in 2010 by researchers at Umeå University and is built on over 50 years of research in the field of lipids. Advancing into the clinical phase marked a significant milestone for all its employees. CEO Stefan K. Nilsson referred to it as the "most crucial step in the company's history."
The study proceeded successfully, with Lipigon subsequently announcing, in October, that Lipisense® exhibits a favorable safety and pharmacokinetic profile and is well-tolerated, with no reports of serious adverse effects. Consequently, Lipigon expanded the SAD study to include a fifth group of healthy volunteers, who were administered a 72 mg dose, in order to explore the maximum tolerability of Lipisense®.
Several Potential Indications
The next phase of the study, the MAD segment, was initiated in parallel with the SAD phase in October 2022, involving 24 participants. The primary goal in this context is to evaluate the safety and tolerability of the drug candidate after four doses of three different dose levels, compared to placebo. The secondary goal is to further assess the triglyceride-lowering effect.
"Potentially, we may gain an added advantage in the Phase I study if we observe a reduction in liver fat content, improved glucose tolerance, and enhanced insulin sensitivity in the study participants. This would indicate that the drug candidate has additional potential indications, particularly for type 2 diabetes," Stefan Pierrou adds.
The development of a potential drug is still several years away, approximately five to six years. The prospect of early data demonstrating effectiveness enhances the likelihood of early out-licensing of a drug with blockbuster potential. Initially, Lipigon focuses on rare diseases that could qualify for orphan drug status or niche indications, potentially reducing time and costs to market entry. There are also good opportunities for market exclusivity and attractive pricing. The drug's mechanism of action suggests that Lipisense® might address a large market for cardiovascular diseases, type 2 diabetes, and fat-related liver diseases. In the focus area, there are significant medical needs, and sales of drugs aimed at regulating blood lipids are expected to reach USD 13.9 billion in 2029.
"Moreover, there are indications that Lipisense® could potentially work for people with obesity, as the drug candidate appears to increase fat burning. This assumption is based on publications where ANGPTL4 in mice has been manipulated in other ways. If we succeed all the way and achieve the meaningful, relevant effects we hope for, there are several large markets here. Most importantly, we could significantly improve the lives of millions of individuals", says Stefan Pierrou.
Read more about Lipigon's projects: